Project
Project
Problem
Space travel takes a serious toll on the human body, and as we look toward a future where life beyond Earth may become a reality, combatting the effects of space on immunity is critical to safeguarding health in the cosmos. In just one week on the International Space Station, astronauts are bombarded with the equivalent of one year’s radiation here on Earth (Radiation and Life, n.d.). Constant exposure to galactic cosmic rays, solar particles from the sun’s solar flares, and radiation trapped in the Earth’s magnetic field creates both acute and long-term effects (Chancellor et al., 2014). Most space radiation is ionizing, meaning it takes the form of high-energy, charged particles. When ionizing radiation enters the body, it interacts with the atoms in our cells, altering our cardiovascular system, inducing cellular ageing, hindering brain function, and breaking and damaging DNA leading to cancer caused by the disrepair of mutations (Chancellor et al., 2014; Space Radiaton, NASA SRAG). Spacesuits and novel detection technologies can only do so much to combat the high levels of radiation exposure astronauts experience. There is a major need in space medicine to help the immune system keep up with the pressures of space and combat excess radiation, with no current products available for astronauts.
We aim to solve this by engineering a DNA origami nanostructure designed to modulate immune responses dynamically; when the DNA nanostructure is triggered, it would modulate a specific response from the immune system to fight radiation.
Background
DNA origami
DNA origami is a technique where a long strand of DNA is folded into specific nanoscale shapes. The technique works by using Watson-Crick base pairing to form specific DNA structures. Unlike the usual double-stranded DNA (dsDNA) found in our cells, DNA origami uses single-stranded DNA (ssDNA) from a bacteriophage genome as a "scaffold strand". Hundreds of synthesised short ssDNA strands, known as “staple strands” or oligonucleotides, hybridise to specific sections of the scaffold strand through base pairing, folding the scaffold into the desired shape (Orponen, 2018; Song et al., 2017). There are several types of DNA origami, the scaffold can be folded into dense 2D brick-like, 3D dense or hollow shapes, or “wireframe” structures, where only the outline of the structure is formed by the double-helix edges (Orponen, 2018; Wagenbauer et al., n.d.). DNA origami has established itself as a potential vector for delivering bioactive molecules and drugs (Singh et al., 2022).
CpG
The immune system is activated in a variety of ways, one of which is through the activation of the innate immune system by unmethylated CpG DNA motifs. A CpG motif is an ssDNA segment containing a cytosine triphosphate deoxynucleotide (‘C’) linked through a phosphodiester (‘p’) to a guanine triphosphate deoxynucleotide (‘G’) which is most commonly found naturally in bacteria. When the CpG is detected by our immune system through toll-like receptor 9, triggering a biosynthetic pathway leading to the production of dendritic cells, cytokines, macrophages and B cells as a part of a Th1-type immune response (Häcker et al., 2002). Put simply, CpG motifs act as a rapid trigger for an inflammatory, anti-infection response from the immune system. Synthetic CpG strands are being explored as an adjuvant for vaccines and immunotherapies; triggering this immune response without any real bacteria could modulate and improve the efficacy of medical products (Klinman, 2004).
Furthermore, DNA origami has proven to be a vessel for CpG oligonucleotides. Zeng et al. (2024) showed that precise spacing of CpG oligonucleotide ligands attached to a DNA origami vaccine triggered this expected reaction. Our solution uses this ability to control adjacent CpG spacing (Zeng et al., 2024) in a DNA origami wireframe structure to help modulate the immune system when the body is exposed to space radiation.
Our Solution
We devised the HubbleBubble, a DNA origami nanostructure inspired by the Hoberman Sphere (Hoberman Associates, n.d.). The device was engineered to transition between open and closed states triggered by stimuli such as radiation or chemical signals (Figure 1). CpG strands attach at the vertices of the structure and upon reconfiguration, the distance between them closes to 3.5 nm of separation (Zeng et al., 2024), triggering an innate immune response. This mechanism is further explained in our Design section.
Future designs could potentially include a trigger strand to dynamically reconfigure the structure according to an input (Gerasimova & Kolpashchikov, 2015; Song et al., 2017). This would make the structure detect excess radiation, triggering a reconfiguration that changes the CpGs’ distance from each other.
Figure 1. The HubbleBubble
The HubbleBubble has merit as an innovative design proof of concept aiming to combine novel immune activation and wireframe DNA origami. It is a complex structure with big therapeutic goals. Considering its potential as a modular design with the capacity to transport adjuvants through the body, the HubbleBubble could be adapted for other therapeutic uses as it is developed.
Goals
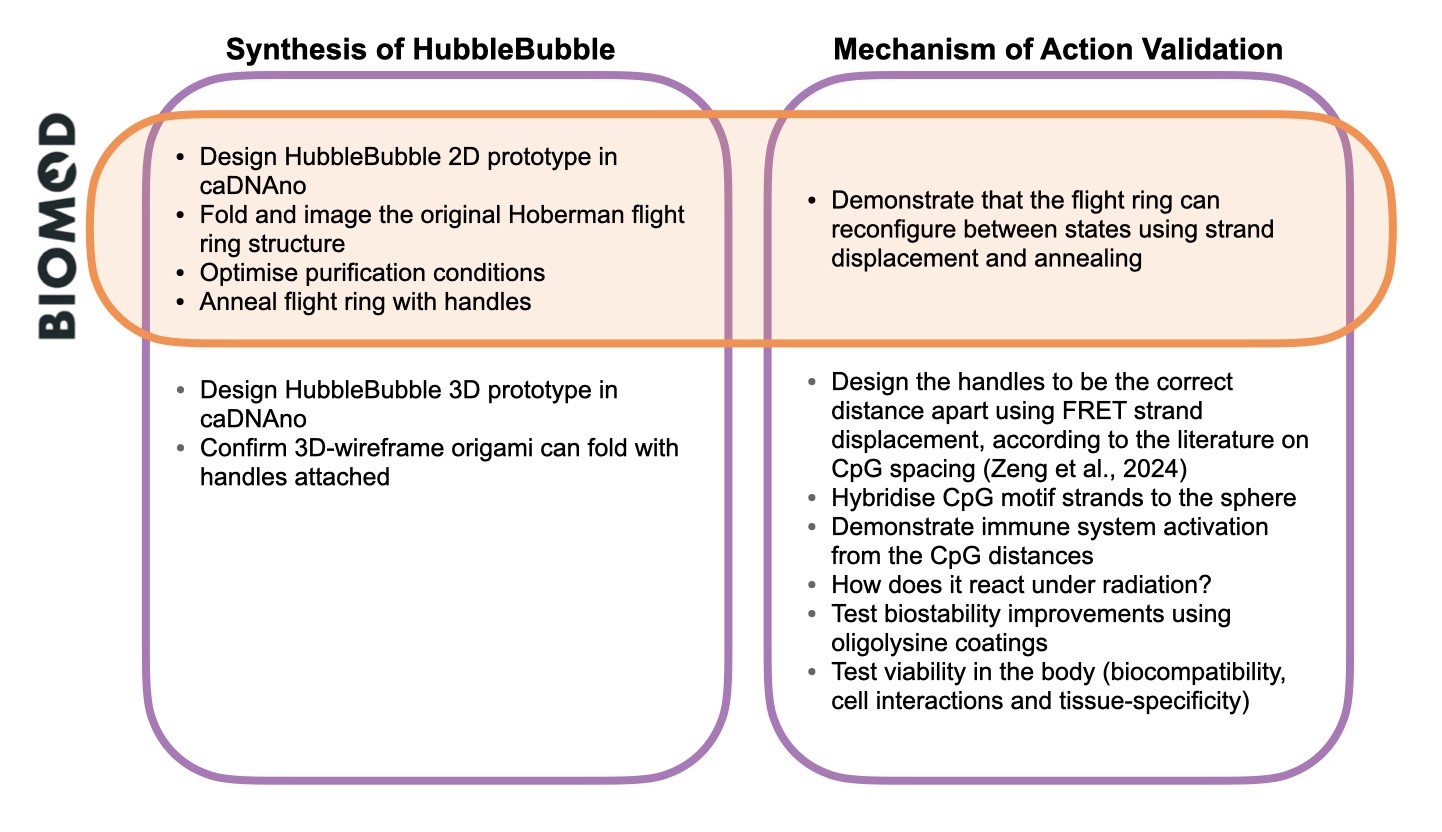
Bringing the HubbleBubble to life requires many experimental stages that cannot be fully completed in the BIOMOD competition’s short time frame. Our main aim for the BIOMOD project is to have a foundational proof of concept for our design. A secondary aim of creating the DNA nanostructure was exploring the feasibility of using the HubbleBubble for in vivo applications.
We organised our project goals into HubbleBubble Synthesis and Mechanism of Action Validation.
Figure 2. BIOMOD Project Goals
Feasibility
Bringing the HubbleBubble to life within the constraints of the BIOMOD competition is nearly impossible; the timeframe and budget are big barriers to successfully creating such a complex structure. That being said, creating a proof of concept is realistic within these limitations. We ensured our goals were feasible by redefining the scope of the project to focus on validating the 2D flight ring structure in caDNAno and in the lab. Choosing the flight ring structure meant we had access to a pre-existing method and design (Li et al., 2017) which we could adapt. We also had the privilege of working within the University of Sydney DNA Nano Group, which gave us access to guidance and resources from DNA origami experts, saving time on trialling methods for basic molecular biology methods like gel electrophoresis.
In terms of the feasibility of the structure itself, the HubbleBubble needs to undergo extensive trials for biostability before going in vivo and inside an astronaut. However, we believe the concept is sound based on the successful reconfiguration of our structures (see Experiment section), and prior literature into both CpG-adjuvant immune regulation and 3D wireframe structures.
Future Work and Usage
Immediate next steps experimentally include optimising purification methods, confirming the CpGs will anneal to the handles and the correct distance, and preparing the flight ring for in vivo testing. Then, converting the principles learned from the 2D flight ring the 3D spherical nanostructure could be tested. The DNA origami will also need to be exposed to space-like radiation.
The HubbleBubble has a range of applications beyond its main purpose of reducing radiation in astronauts’ bodies. Here on Earth, the device could be used for a range of immunotherapeutics using the CpG properties (Klinman, 2004). Furthermore, CpG could be swapped for bio-reactive chemicals, or the open/closed reconfiguration could be used for drug particle delivery.
References
Chancellor, J. C., Scott, G. B. I., & Sutton, J. P. (2014). Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life : Open Access Journal, 4(3), 491. https://doi.org/10.3390/life4030491
Gerasimova, Y. V., & Kolpashchikov, D. M. (2015). Divide and control: Split design of multi-input DNA logic gates. Chemical Communications (Cambridge, England), 51(5), 870–872. https://doi.org/10.1039/c4cc08241a
Häcker, G., Redecke, V., & Häcker, H. (2002). Activation of the immune system by bacterial CpG-DNA. Immunology, 105(3), 245. https://doi.org/10.1046/j.0019-2805.2001.01350.x
Hoberman Associates. (n.d.). Hoberman Associates. Retrieved November 14, 2024, from https://www.hoberman.com/
Klinman, D. M. (2004). Immunotherapeutic uses of CpG oligodeoxynucleotides. Nature Reviews Immunology, 4(4), 249–259. https://doi.org/10.1038/nri1329
Li, R., Chen, H., & Choi, J. H. (2021). Topological Assembly of a Deployable Hoberman Flight Ring from DNA. Small, 17(11), 2007069. https://doi.org/10.1002/smll.202007069
Orponen, P. (2018). Design methods for 3D wireframe DNA nanostructures. Natural Computing, 17(1), 147–160. https://doi.org/10.1007/s11047-017-9647-9
Ponnuswamy, N., Bastings, M. M. C., Nathwani, B., Ryu, J. H., Chou, L. Y. T., Vinther, M., Li, W. A., Anastassacos, F. M., Mooney, D. J., & Shih, W. M. (2017). Oligolysine-based coating protects DNA nanostructures from low-salt denaturation and nuclease degradation. Nature Communications, 8(1), 15654. https://doi.org/10.1038/ncomms15654
Radiation and life. (n.d.). European Space Agency. Retrieved November 14, 2024, from https://www.esa.int/Science_Exploration/Human_and_Robotic_Exploration/Lessons_online/Radiation_and_life
Singh, M., Sharma, D., Garg, M., Kumar, A., Baliyan, A., Rani, R., & Kumar, V. (2022). Current understanding of biological interactions and processing of DNA origami nanostructures: Role of machine learning and implications in drug delivery. Biotechnology Advances, 61, 108052. https://doi.org/10.1016/j.biotechadv.2022.108052
Song, J., Li, Z., Wang, P., Meyer, T., Mao, C., & Ke, Y. (2017). Reconfiguration of DNA molecular arrays driven by information relay. Science, 357(6349), eaan3377. https://doi.org/10.1126/science.aan3377
Space Radiation: An Important Concern for Human Spaceflight. (NASA SRAG). NASA - Space Radiation Analysis Group (SRAG) Web Site; Terrie Bevill. https://srag.jsc.nasa.gov/SpaceRadiation/Why/Why.cfm
Wagenbauer, K. F., Engelhardt, F. A. S., Stahl, E., Hechtl, V. K., Stömmer, P., Seebacher, F., Meregalli, L., Ketterer, P., Gerling, T., & Dietz, H. (n.d.). How We Make DNA Origami. https://doi.org/10.1002/cbic.201700377
Zeng, Y. C., Young, O. J., Wintersinger, C. M., Anastassacos, F. M., MacDonald, J. I., Isinelli, G., Dellacherie, M. O., Sobral, M., Bai, H., Graveline, A. R., Vernet, A., Sanchez, M., Mulligan, K., Choi, Y., Ferrante, T. C., Keskin, D. B., Fell, G. G., Neuberg, D., Wu, C. J., … Shih, W. M. (2024). Fine tuning of CpG spatial distribution with DNA origami for improved cancer vaccination. Nature Nanotechnology, 19(7), 1055–1065. https://doi.org/10.1038/s41565-024-01615-3